Researchers study the adsorption characteristics of fluorinated covalent organic polymers against environmentally persistent pharmaceuticals
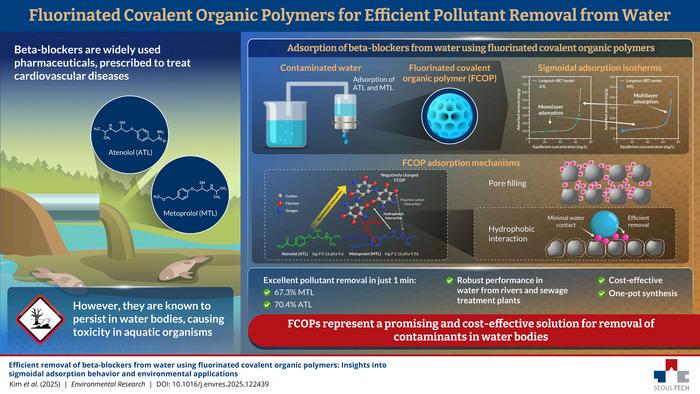
Beta-blockers are commonly prescribed medications for managing cardiovascular issues such as hypertension, arrhythmias, and recovery following heart attacks. Among these, atenolol (ATL) and metoprolol (MTL) are widely used. While their chemical stability ensures effective therapeutic action, it also means they persist in the environment. Conventional wastewater treatment plants struggle to remove these compounds, allowing even low concentrations to accumulate in rivers and lakes, where they can cause chronic toxicity in aquatic organisms like algae and fish.
To address the challenge of removing these persistent pharmaceuticals, researchers have turned to advanced adsorbent materials such as covalent organic polymers (COPs). These porous materials can be tailored with diverse functional groups, enabling precise control over adsorption properties. Recently, COPs enriched with fluorine atoms have attracted attention due to their exceptional adsorption performance, yet their potential in removing pharmaceuticals has been largely unexplored.
Filling this knowledge gap, a team led by Professor Yuhoon Hwang from the Department of Environmental Engineering at Seoul National University of Science and Technology (SeoulTech) investigated fluorine-rich COPs (FCOPs) as adsorbents for beta-blockers. “Our results indicate that FCOPs offer a highly effective solution for eliminating persistent beta-blockers from water,” notes Prof. Hwang. “We also uncovered the adsorption mechanisms responsible for their unusually high capacity.” The study was first made available online on July 28, 2025, and later published in Environmental Research (Volume 285, Part 3, November 15, 2025).
The researchers synthesized FCOPs using a straightforward, catalyst-free one-pot method and tested their ability to remove ATL and MTL from water. The results were striking: within just one minute, the FCOPs removed 70.4% of ATL and 67.3% of MTL. When adsorption performance was plotted against beta-blocker concentration, the curve displayed a characteristic sigmoidal (S-shaped) profile.
At lower concentrations, adsorption increased gradually, consistent with monolayer adsorption, where molecules form a single layer on the adsorbent surface. Beyond 60 mg/L, however, uptake surged sharply for both drugs, indicating multilayer adsorption. In this regime, molecules stack in multiple layers, enhancing adsorption through mutual interactions. This distinctive behavior sets FCOPs apart from conventional adsorbents. Remarkably, the FCOPs retained this high adsorption efficiency even in complex real water samples containing multiple ions and organic acids.
The team also investigated the mechanisms behind this exceptional performance. The fluorine-rich structure of FCOP enables multiple synergistic interactions: strong intermolecular interactions between FCOP and beta-blockers, electrostatic attraction of the positively charged drugs to the negatively charged polymer, and hydrophobic effects that promote molecule aggregation and facilitate multilayer adsorption.
“These combined mechanisms explain the extraordinary adsorption capacity of FCOPs,” Prof. Hwang explains. “Our findings lay the groundwork for designing next-generation adsorbents. In the future, fluorine-rich materials like these could play a crucial role in reducing pharmaceutical contamination, protecting aquatic ecosystems, and ensuring safer drinking water.”
By incorporating FCOPs into advanced water treatment systems, utilities could more effectively mitigate pharmaceutical pollution. This approach represents a promising step toward sustainable water purification strategies that safeguard both human health and the environment.
Source & Image: https://bit.ly/496fgFI